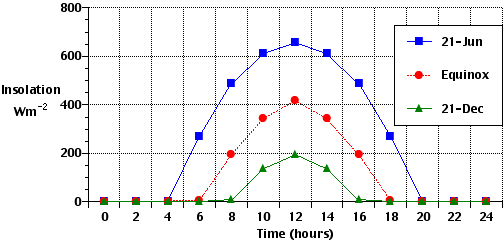
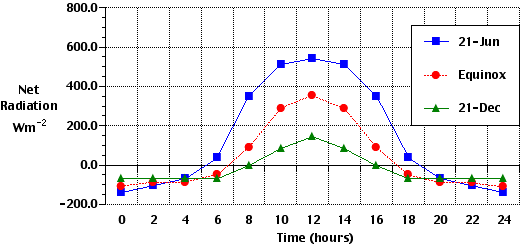
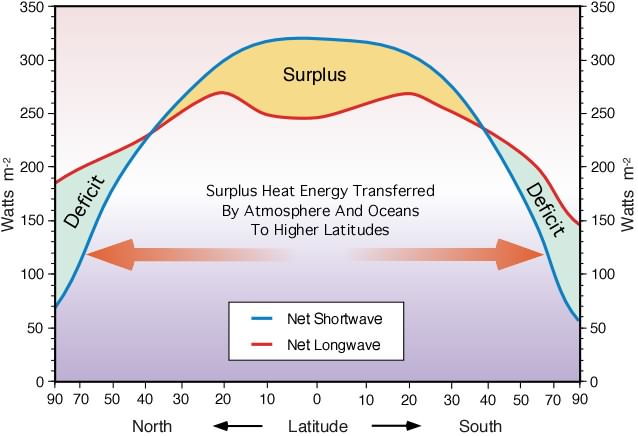
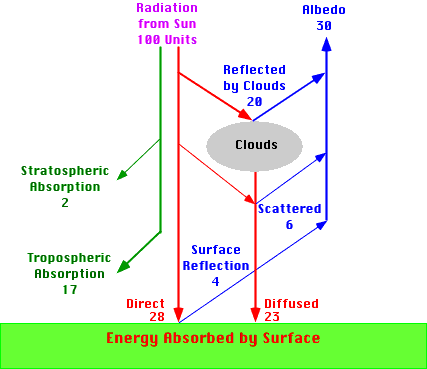
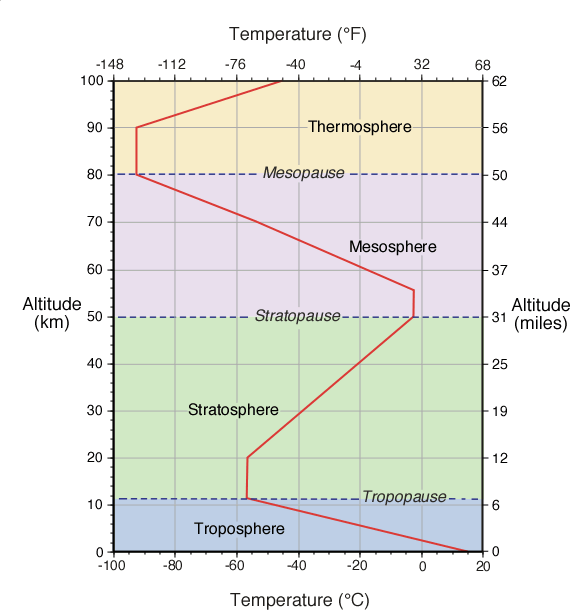
The Earth's atmosphere contains several different layers that can be defined according to air temperature, Figure 7b-1 displays these layers in an average atmosphere.
Figure 7b-1: Vertical change in average global atmospheric temperature. Variations in the way temperature changes with height indicates the atmosphere is composed of a number of different layers (labeled above). These variations are due to changes in the chemical and physical characteristics of the atmosphere with altitude.
According to temperature, the atmosphere contains four different layers (Figure 7b-1). The first layer is called the troposphere. The depth of this layer varies from about 8 to 16 kilometers. Greatest depths occur at the tropics where warm temperatures causes vertical expansion of the lower atmosphere. From the tropics to the Earth's polar regions the troposphere becomes gradually thinner. The depth of this layer at the poles is roughly half as thick when compared to the tropics. Average depth of the troposphere is approximately 11 kilometers as displayed in Figure 7b-1.
About 80% of the total mass of the atmosphere is contained in troposphere. It is also the layer where the majority of our weather occurs (Figure 7b-2). Maximum air temperature also occurs near the Earth's surface in this layer. With increasing height, air temperature drops uniformly with altitude at a rate of approximately 6.5° Celsius per 1000 meters. This phenomenon is commonly called the Environmental Lapse Rate. At an average temperature of -56.5° Celsius, the top of the troposphere is reached. At the upper edge of the troposphere is a narrow transition zone known as the tropopause.
Figure 7b-2: Most of our planet's weather occurs in the troposphere. This image shows a view of this layer from an airplane's window (Photo © 2004 Edward Tsang).
Above the tropopause is the stratosphere. This layer extends from an average altitude of 11 to 50 kilometers above the Earth's surface. This stratosphere contains about 19.9% of the total mass found in the atmosphere. Very little weather occurs in the stratosphere. Occasionally, the top portions of thunderstorms breach this layer. The lower portion of the stratosphere is also influenced by the polar jet stream and subtropical jet stream. In the first 9 kilometers of the stratosphere, temperature remains constant with height. A zone with constant temperature in the atmosphere is called an isothermal layer.
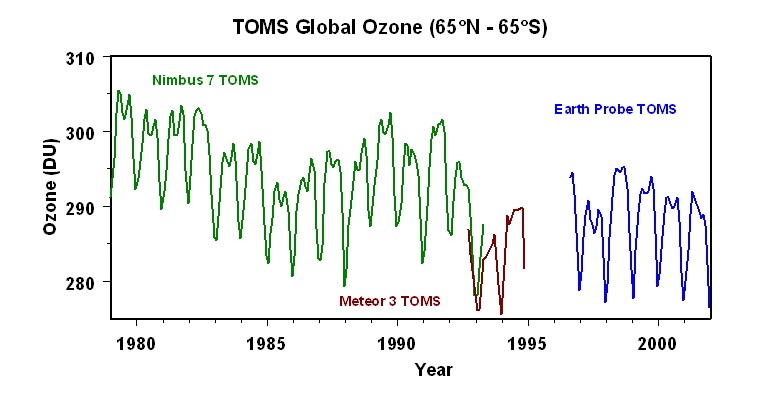
From an altitude of 20 to 50 kilometers, temperature increases with an increase in altitude. The higher temperatures found in this region of the stratosphere occurs because of a localized concentration of ozone gas molecules. These molecules absorb ultraviolet sunlight creating heat energy that warms the stratosphere. Ozone is primarily found in the atmosphere at varying concentrations between the altitudes of 10 to 50 kilometers. This layer of ozone is also called the ozone layer . The ozone layer is important to organisms at the Earth's surface as it protects them from the harmful effects of the Sun's ultraviolet radiation. Without the ozone layer life could not exist on the Earth's surface.
Separating the mesosphere from the stratosphere is transition zone called the stratopause. In the mesosphere, the atmosphere reaches its coldest temperatures (about -90° Celsius) at a height of approximately 80 kilometers. At the top of the mesosphere is another transition zone known as the mesopause.
The last atmospheric layer has an altitude greater than 80 kilometers and is called the thermosphere. Temperatures in this layer can be greater than 1200° C. These high temperatures are generated from the absorption of intense solar radiation by oxygen molecules (O2). While these temperatures seem extreme, the amount of heat energy involved is very small. The amount of heat stored in a substance is controlled in part by its mass. The air in the thermosphere is extremely thin with individual gas molecules being separated from each other by large distances.
Consequently, measuring the temperature of thermosphere with a thermometer is a very difficult process. Thermometers measure the temperature of bodies via the movement of heat energy. Normally, this process takes a few minutes for the conductive transfer of kinetic energy from countless molecules in the body of a substance to the expanding liquid inside the thermometer. In the thermosphere, our thermometer would lose more heat energy from radiative emission then what it would gain from making occasional contact with extremely hot gas molecules.
CITATION
Pidwirny, M. (2006). "The Layered Atmosphere". Fundamentals of Physical Geography, 2nd Edition. Date Viewed. http://www.physicalgeography.net/fundamentals/7b.html